
Atomic number, 92 atomic weight, 238.029. U, a radioactive chemical element of group III of Mendeleev’s periodic system a member of the actinide series.

Because the supply of uranium-235 is limited, countries have worked to develop fast breeder reactors that convert nonfissionable uranium-238 to fissionable plutonium-239 (see nuclear reactor).
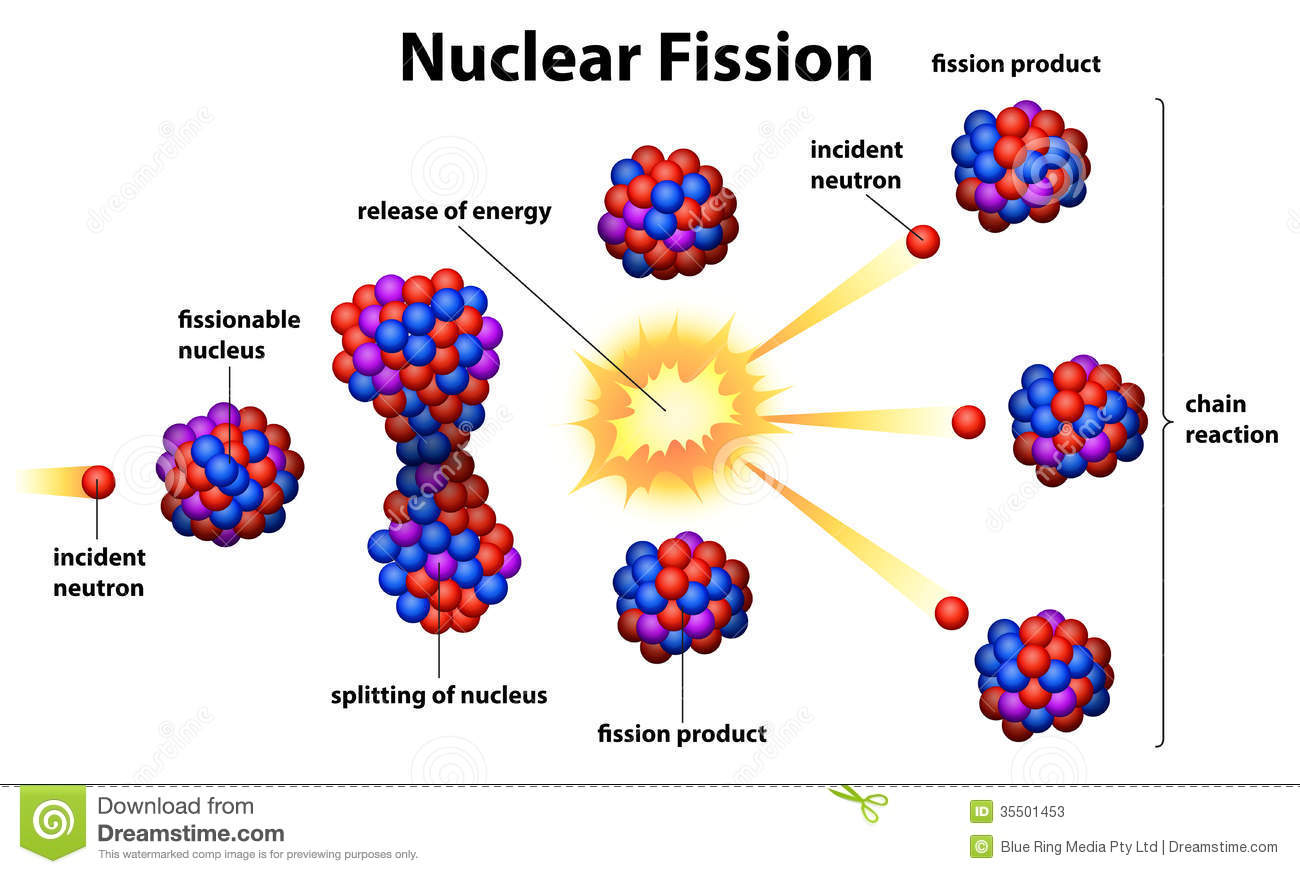
Uranium-235 is the only naturally occurring nuclear fission fuel, but this isotope is only about 1 part in 140 of natural uranium the balance is mostly uranium-238. Uranium gained importance with the development of practical uses of nuclear energy. However, because of the high toxicity (both chemical and radiological) of uranium and its compounds, and because of their importance as nuclear fuel, these earlier uses have been largely curtailed. It has also been added to steels to increase their strength and toughness. Before the discovery of nuclear fission by Otto Hahn and Fritz Strassmann in 1939, the principal use of uranium (chiefly as the oxides) was in pigments, ceramic glazes, and a yellow-green fluorescent glass and as a source of radium for medical purposes. Becquerel discovered its radioactivity in 1896. However, the substance that Klaproth identified was not pure uranium but an oxide. Klaproth, who in 1789, while experimenting with pitchblende, concluded that it contained a new element, which he named after the planet Uranus, discovered only eight years earlier. The discovery of uranium is commonly credited to Martin H. In enrichment, uranium-238 is separated from uranium-235 by a diffusion or centrifuge process using the gaseous hexafluoride, UF 6, which is produced when the metal reacts with fluorine. Uranium is further processed, or enriched, to increase the percentage of uranium-235 so that the uranium can be used in a reactor or, with much greater enrichment, a weapon. The pure metal is obtained by electrolysis or chemical reduction of the tetrafluoride, or by chemical reduction of the dioxide. The dioxide may be converted to the tetrafluoride, UF 4, by treatment with hydrogen fluoride gas, HF. The dioxide is chemically and physically stable at high temperatures, and is the form most often used as nuclear reactor fuel. The uranium is obtained as pure uranyl nitrate, UO 2(NO 3) 2♶H 2O, which is typically decomposed to the trioxide, UO 3, by heating and reduced to the dioxide, UO 2, with hydrogen. Leaching produces the material known as yellowcake, which is largely triuranium octoxide (U 3O 8) it is then processed with nitric acid. Most ores are processed by chemical methods including leaching and solvent extraction. Ores with as little as 0.1% uranium are mined and processed. The most common are uraninite (essentially uranium dioxide) and pitchblende other commercially important uranium-containing minerals include carnotite (a potassium uranate-vanadate) and brannerite (a uranium titanate). Several hundred uranium-containing minerals have been found but only a few are commercially significant.
It is a fairly abundant element in the earth's crust, being about 40 times as abundant as silver. Uranium is widely distributed in its ores but is not found uncombined in nature.
#Fission uranium buyout series
Because the rate of decay in these series is constant, it is possible to estimate the age of uranium samples (e.g., minerals) from the relative amounts of parent substance and final product (see dating). Uranium-235, also called actinouranium, is the parent substance of the so-called actinium series, a 15-member radioactive decay series ending in stable lead-207 protactinium-231 and actinium-227 are the relatively stable members of this series. Some relatively long-lived members of this series include uranium-234, thorium-230, and radium-226 the final stable member of the series is lead-206. Uranium-238 is the parent substance of the 18-member radioactive decay series known as the uranium series (see radioactivity). The most abundant (greater than 99%) and most stable is uranium-238 ( half-life 4.5×10 9 years) also present are uranium-235 (half-life 7×10 8 years) and uranium-234 (half-life 2.5×10 5 years). Naturally occurring uranium is a mixture of three isotopes.


 0 kommentar(er)
0 kommentar(er)
